A stable low-temperature H2-production catalyst by crowding Pt on α-MoC published in Nature
DOI: 10.1038/s41586-020-03130-6
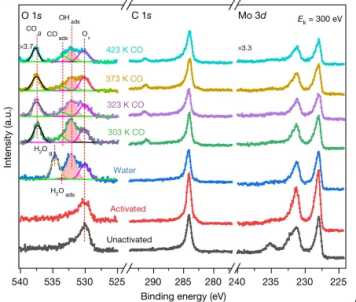
The water–gas shift reaction, leading to the production of hydrogen and carbon dioxide from carbon monoxide and water, is of paramount industrial importance. High activity and stability of the catalysts, typically employed in fuel cells, are important parameter to consider to properly design new materials.
In this work, combining reactivity tests with microscopy and spectroscopy, it has been demonstrated that the co-presence of platinum isolated atoms and nanoclusters supported on molybdenum carbide allows the protection of the support, leading to high stability and high metal-normalized turnover number of 4,300,000 moles of hydrogen per mole of platinum.
More information can be accessed online by clicking on the following link: external page paper in Nature