Watching transformation of platinum catalysts live and with atomic resolution
A recent paper (DOI: 10.1038/s41467-020-17070-2), result of a collaboration of our group with the Scientific Center for Optical and Electron Microscopy (ScopeM), provides a novel real time view on how the strong metal-support interaction (SMSI) phenomenon occurs.
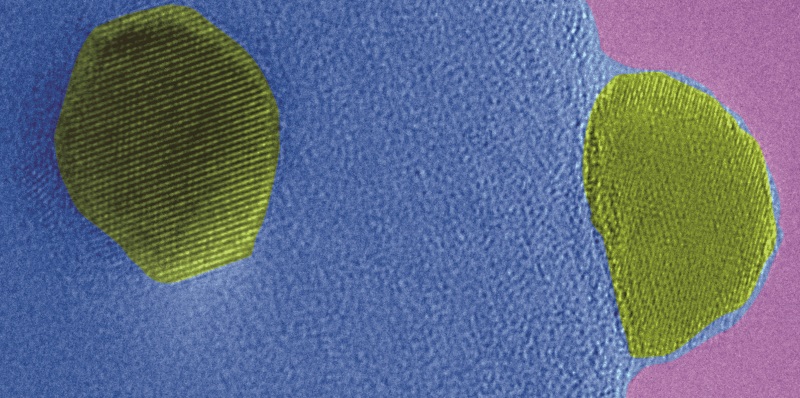
Heterogeneous catalysts are a key of the chemical industry. More than 80% of all produced goods have a catalyst involved during their production. To understand and improve catalysts is necessary for a transition to an environmentally friendly society, by making existing processes energy efficient and enabling new technology routes like fuel cells. Platinum is widely used as catalytic material, but the platinum resources are limited on our planet. Tuning the properties of platinum catalysts to high performance is important to make optimal use of this scarce metal. Therefore, platinum in usually distributed as very small nanoparticles (1-10 nm) on metal oxide support materials. For metal oxides that are easy to reduce (like titania), a treatment in hydrogen can alter the structure and performance of the catalyst making it more efficient for specific catalytic reactions1–3. These changes occur because tiny layers of the support material form on top of the platinum particles. More than four decades ago this phenomenon was initially described3. However, still little understanding of this phenomenon exists, because very often the catalysts is only studied after it was removed from the reactor. Removing a sample from the conditions in which it is treated, changes the sample. It is cooled down and for example hydrogen is removed. That means what you see after the process, is most likely not what it was like during the process.
Therefore, in a recent work4, the group of Prof. Jeroen van Bokhoven from the Institute for Chemical and Bioengineering of ETH Zurich and the Paul Scherrer Institute, together with Xing Huang and Marc-Georg Willinger from the Scientific Center for Optical and Electron Microscopy (ScopeM), used methods that are able to study the catalyst directly during the process when the overlayer over the platinum is formed. Especially, state-of-the-art transmission electron microscopy was employed to see the overlayer formation with atomic resolution and time resolution while it is formed. The recorded movies unraveled new and important insights into the process of overlayer formation. The researchers found that during overlayer formation also a platinum-titanium surface alloy forms and that these two processes are competing. Moreover, it was found that the overlayer is as well stable in oxygen under high temperatures, which was previously unknown. The findings where complemented with X-ray spectroscopy, X-ray diffraction, and theoretic calculation to validate the microscopy findings and derive further detail information to paint a holistic picture of the processes that occur in the catalyst.
The role of oxygen in the formation process and stabilization of the overlayer was not recognized before. The stabilization of the oxide overlayer in oxygen environment shows that this overlayer can as well be utilized in catalytic oxidation reactions. This path so far lacks of intense study and requires attention from the scientific community. As shown in the work, the combination of emerging in situ techniques enables to derive a complimentary and holistic view on materials under conditions that are important for our understanding of phenomena that happen under harsh process conditions.
More information can be accessed online by clicking on the following links: Download paper in Nature Communications and Download Kudos.
References
- Matsubu, J. C. et al. Adsorbate-mediated strong metal–support interactions in oxide-supported Rh catalysts. Nat. Chem. 9, 120–127 (2016).
- Corma, A., Serna, P., Concepción, P. & Calvino, J. J. Transforming nonselective into chemoselective metal catalysts for the hydrogenation of substituted nitroaromatics. J. Am. Chem. Soc. 130, 8748–8753 (2008).
- Tauster, S. J., Fung, S. C. & Garten, R. L. Strong Metal-Support Interactions. Group 8 Noble Metals Supported on TiO2. J. Am. Chem. Soc. 100, 170–175 (1978).
- Beck, A. et al. The dynamics of overlayer formation on catalyst nanoparticles and strong metal-support interaction. Nat. Commun. 11, 3220 (2020).